Marietta, Ohio, April 6, 2020
ProtoKinetix, Incorporated (www.protokinetix.com) (the “Company” or “ProtoKinetix”) (OTCQB:PKTX), a clinical-stage biomedical company, having recently announced filing for patent protection for new applications of its AAGP® (PKX-001) molecule, announces preliminary results in efficacy and safety for use in treating Dry Eye Disease (DED).
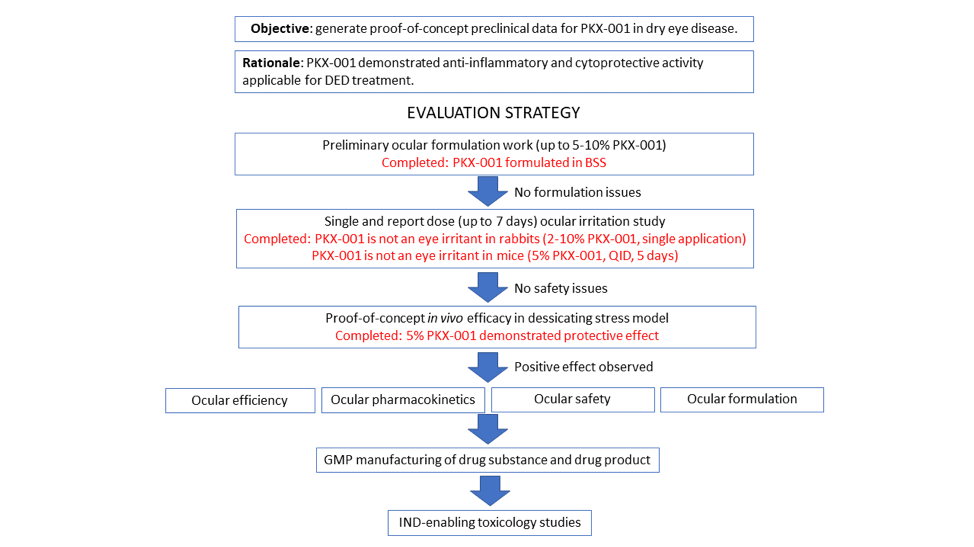
PKX-001 demonstrates a protective effect in the murine model of dry eye disease. The effect of 5% PKX-001, delivered QID via bilateral topical administration, was evaluated in an acute murine model of desiccating stress (DS). The study was conducted by EyeCRO LLC (Oklahoma City, US). Corneal permeability was assessed by Oregon Green Dextran (OGD). CsA-MiDROPS™ significantly reduced DS-induced corneal permeability. PKX-001 reduced the DS-induced corneal permeability to the same level as the positive control CsA-MiDROPS. Additional tests are currently ongoing.
PKX-001 has a favorable in vitro and in vivo safety profile. PKX-001 safety upon ocular administration was evaluated in two animal models. The first study was conducted by ITR Laboratories (Montreal, Canada). PKX-001 formulated in BSS at a concentration up to 10% was not considered an eye irritant.
The second study was performed by EyeCRO LLC (Oklahoma City, US). Treated eyes were scored for chemosis, hyperemia and discharge on a daily basis. PKX-001 was well tolerated and not considered an eye irritant.
Furthermore, PKX-001 was negative in the bacterial reverse mutation assay and did not induce chromosomal damage in the micronucleus test in animal model ovary cells (ITR laboratories, Montreal, Canada).
PKX-001 demonstrated anti-inflammatory and cytoprotective properties:
- Reduced levels of anti-inflammatory cytokines
- Decreased oxidative stress
- Increased cellular survival and improved functional activity under various stress conditions
“These positive results in efficacy and safety in the recent testing of our AAGP® for dry eye disease is extremely promising for the future of using our molecule to treat this disease.” – said Clarence E. Smith, President and Chief Executive Officer of ProtoKinetix. “We are currently planning to move forward with the next stages of development ourselves.”
Market Overview
According to market research published by Mordor Intelligence LLP, studies of the Dry Eye Disease market indicated a value of approximately USD 4.5 billion in 2018, and the market is expected to reach up to USD 6.2 billion by 2024, with an anticipated CAGR of 5.23%, during the forecast period (2019-2024). The growth of dry eye related diseases may include several factors, such as aging, a decrease in the supportive hormones, systemic inflammatory diseases, ocular surfaces diseases or surgeries affecting the cholinergic nerves, which stimulate tear secretion.
Dry Eye Disease is one of the most common ocular problems with an estimated prevalence of almost five million people over the age of 50 in the United States alone. Cyclosporine A is the first prescription product for dry eye therapy, which increases tear production in patients whose tear production is suppressed. However, Cyclosporine A treatment presents disadvantages over the long term which could potentially be mitigated by AAGP®.
About ProtoKinetix, Incorporated
ProtoKinetix is a molecular biotechnology company that has developed and patented a family of hyper stable, potent glycopeptides (AAGP®) that enhance both engraftment and protection of transplanted cells, tissues and organs used in regenerative medicine. Due to the results achieved over the last four years of testing, the University of Alberta has begun Phase 1 human clinical trials. Additional studies will be expanded to include whole organ transplantation and all therapies that are being developed globally to date; diabetes, retinal degeneration, cardiac repair and many other degenerative conditions. In addition, we are studying the potential impact on several cancer therapies.
Cautionary Note Regarding Forward-Looking Statements
The information discussed in this press release includes “forward looking statements” within the meaning of Section 27A of the Securities Act of 1933 (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”). All statements, other than statements of historical facts, included herein concerning, among other things, planned capital expenditures, future cash flows and borrowings, pursuit of potential acquisition opportunities, our financial position, business strategy and other plans and objectives for future operations, are forward looking statements. These forward looking statements are identified by their use of terms and phrases such as “may,” “expect,” “estimate,” “project,” “plan,” “believe,” “intend,” “achievable,” “anticipate,” “will,” “continue,” “potential,” “should,” “could,” and similar terms and phrases. Although we believe that the expectations reflected in these forward looking statements are reasonable, they do involve certain assumptions, risks and uncertainties and are not (and should not be considered to be) guarantees of future performance. Among these risks are those set forth in a Form 10-K filed on February 19, 2020. It is important that each person reviewing this release understand the significant risks attendant to the operations of ProtoKinetix. ProtoKinetix disclaims any obligation to update any forward-looking statement made here.
This press release does not constitute or form a part of any offer or solicitation to purchase or subscribe for securities in the United States. The securities referred to herein have not been and will not be registered under the Securities Act of 1933, as amended (the “Securities Act”), or with any securities regulatory authority of any state or other jurisdiction in the United States, and may not be offered or sold, directly or indirectly, except pursuant to an exemption from or in a transaction not subject to the registration requirements of the Securities Act.
For further information, please contact:
Clarence E. Smith
President and chief executive officer
Telephone: 740-434-5041
Email: [email protected]
Twitter: @ProtoKinetix