October 2007
Introduction
The purpose of this preliminary evaluation is to better understand the protective properties of AAGP®.
Method
Umbilical cord blood was processed in parallel using standard independent 3rd party procedures with cords being split between two cryoprotectant media options, one consisting of our standard media and one consisting of our standard media supplemented with 4.0-4.4 mg/mL AAGP®. Each split was analyzed post-processing yet pre-freeze to determine total and progenitor cell counts. The units were frozen via standard protocol with a 2°C/min freezing profile to an intermediate temperature of -80°C. The units were then transferred to -196°C in LN2 and stored for an unspecified timeframe. On thawing the units were evaluated for viability, total cell content (TNC), CD34+ progenitor cell content, recovery of TNC and CD34+ cells, and functionality of stem and progenitor activity through Colony Forming Unit (CFU) assay.
Results
Results showed the presence of AAGP® in the freeze media to have an overall position affect, though slight, on the percentage of total cell count recovery. However, the most impressive results were found for percentage recovery of CD34+ cells where presence of AAGP® increased recovery by up to 21%. The percentage of functional cells, as assessed by CFU assay, was also increased by up to 39% in the presence of AAGP®.
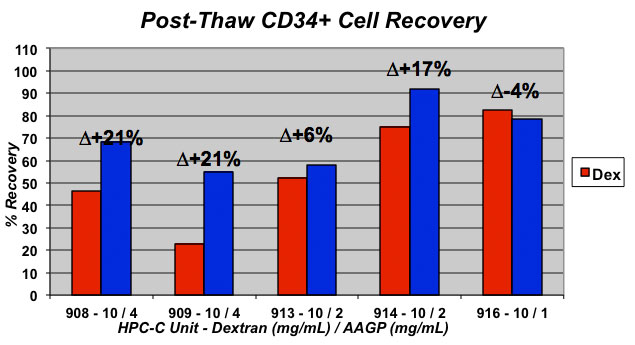
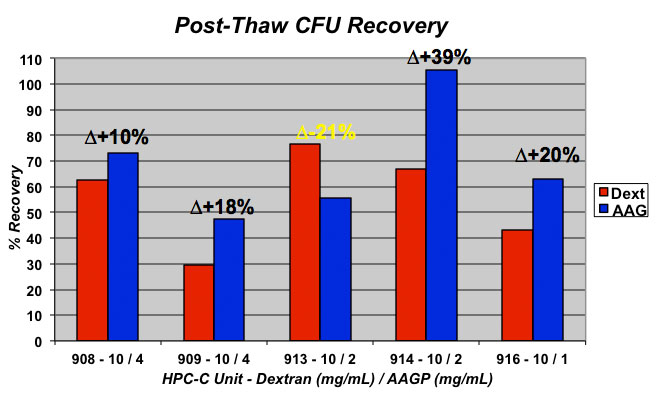
Conclusion
These results suggest AAGP® to be a valuable agent in increasing cell survival and functionality of cord blood cells during freezing.