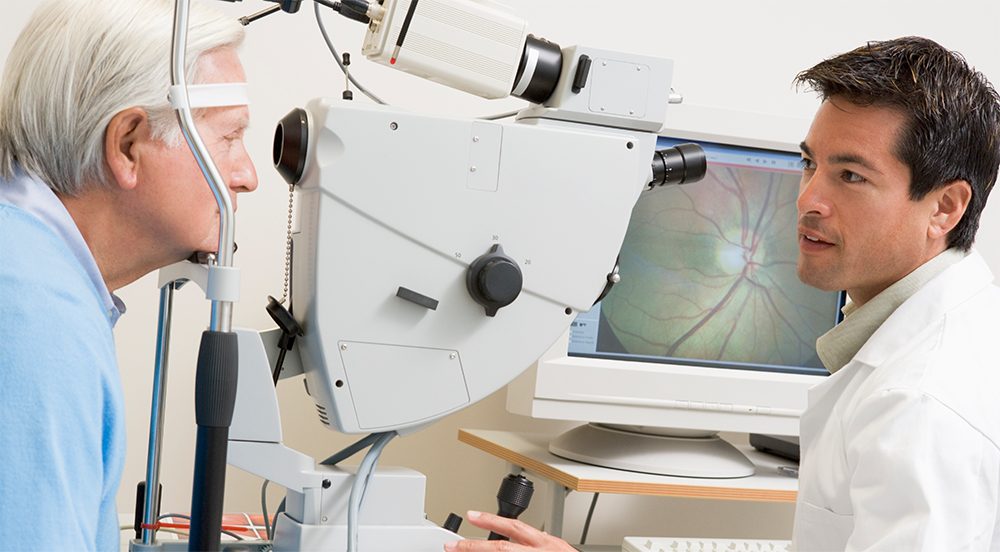
ProtoKinetix, Incorporated (www.protokinetix.com) (the “Company” or “ProtoKinetix”) (OTCQB:PKTX) updates shareholders regarding ongoing 3rd stage testing of retinal cell replacement therapy at the University of British Columbia. As previously reported, the Company has demonstrated the ability of AAGP® to protect transplanted cells in a pre-clinical experimental model of retinal degeneration. For the purpose of these tests the animals had a functioning immune system and so were treated with immune modulating drugs to model clinical practices. The results clearly demonstrated the ability of AAGP® to protect the delicate transplanted cells from the stress of the local microenvironment after transplant into the recipient at the four-week timepoint. Two questions under study have been answered:
- 1. Do the transplanted cells survive in the recipient and safely continue to mature into functional retinal cells?
- 2. Does AAGP® interfere with the fated development of the transplanted cells?
In order to answer these essential questions a comprehensive series of tests using immune suppressed animal models were designed. These tests included a long-term follow-up out to six months to determine if the cells continued to mature into photo-sensitive cells and whether the presence of AAGP® interfered with this essential development. At the five-month timepoint the tests show that AAGP® preserved and allowed these cells to mature without compromise.
Pluripotent stem-cell therapy guided into retinal cells could potentially cure blindness even in the late stages of disease. However, until now, studies in animals have shown that too few transplanted retinal cells survive the hostile local environment long enough to integrate correctly into the retina’s complex neural circuitry. The AAGP® molecule in this study has overcome this considerable obstacle for stem-cell treatments that aim to replace retinal cells.
These studies are a critical component of the pre-clinical testing required to advance this program into clinical trials. The study is being conducted by the Gregory-Evans Retinal Therapeutic Lab at the University of British Columbia.
We are now completing functional studies in two different animal models. These include electrical responses of the eye and also a behavioral test of sharpness of vision. Preliminary results show retention of vision function particularly in behavioral testing in the rodent model. Also, of note, we have not documented any adverse effects in animals when using AAGP®. Although our results are in relatively small numbers of animals (a dozen in each cohort of testing) this bodes exceptionally well for any proposed future clinical trial work.
Cautionary Note Regarding Forward-Looking Statements
The information discussed in this press release includes “forward looking statements” within the meaning of Section 27A of the Securities Act of 1933 (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”). All statements, other than statements of historical facts, included herein concerning, among other things, planned capital expenditures, future cash flows and borrowings, pursuit of potential acquisition opportunities, our financial position, business strategy and other plans and objectives for future operations, are forward looking statements. These forward looking statements are identified by their use of terms and phrases such as “may,” “expect,” “estimate,” “project,” “plan,” “believe,” “intend,” “achievable,” “anticipate,” “will,” “continue,” “potential,” “should,” “could,” and similar terms and phrases. Although we believe that the expectations reflected in these forward looking statements are reasonable, they do involve certain assumptions, risks and uncertainties and are not (and should not be considered to be) guarantees of future performance. Among these risks are those set forth in a Form 10-K filed on March 12, 2019. It is important that each person reviewing this release understand the significant risks attendant to the operations of ProtoKinetix. ProtoKinetix disclaims any obligation to update any forward-looking statement made here.
This press release does not constitute or form a part of any offer or solicitation to purchase or subscribe for securities in the United States. The securities referred to herein have not been and will not be registered under the Securities Act of 1933, as amended (the “Securities Act”), or with any securities regulatory authority of any state or other jurisdiction in the United States, and may not be offered or sold, directly or indirectly, except pursuant to an exemption from or in a transaction not subject to the registration requirements of the Securities Act.
Clarence E. Smith
President and Chief Executive Officer
Learn more about ProtoKinetix or contact us to discuss potential investment opportunities.